
Pandemic disruption and a new MA code update; what for patient programs in this new era?
COVID-19 has taught us that in times of uncertainty, people seek information and…


Pandemic disruption and a new MA code update; what for patient programs in this new era?
COVID-19 has taught us that in times of uncertainty, people seek information and…

Hand sanitiser – What do you need to know to supply in Australia?
The COVID-19 pandemic has seen the TGA rapidly changing the regulatory landscape for in-demand products that can help reduce the spread of the virus. Given all the changes taking place,…

(NOT) BUSINESS AS USUAL
At Commercial Eyes, we focus our efforts on supporting clients in the regulated therapeutics industry get their products to the patients who need them, when they need…

Brexit – What’s the impact on medicines and medical devices supplied in Australia?
A transition period is now in effect until the end of 2020 with the possibility for extension. Its status quo for Australia during the transition period, with the current regulations for…

ARCS Australia appoints Commercial Eyes Founder and Managing Director to Board.
ARCS Australia has appointed Commercial Eyes Founder and Managing Director, Andrew Carter, to its Board of…

Commercial Eyes at PharmAus19
It is a privilege for the Commercial Eyes team to work closely with so many of the companies represented at PharmAus, to play a key role in making their innovations…

Educating and empowering Indigenous children through Yalari
Commercial Eyes is honoured to be able to provide ongoing support to educating Indigenous children through…

Leukaemia Foundation – Raising Awareness of Blood Cancers
Through various parts of our business including market access and insights, regulatory services and medical communications, Commercial Eyes works with a number of organisations involved in improving and extending the…
No CE mark? No problem.
Changes to legislation have increased flexibility for Sponsors looking to market medical devices in…
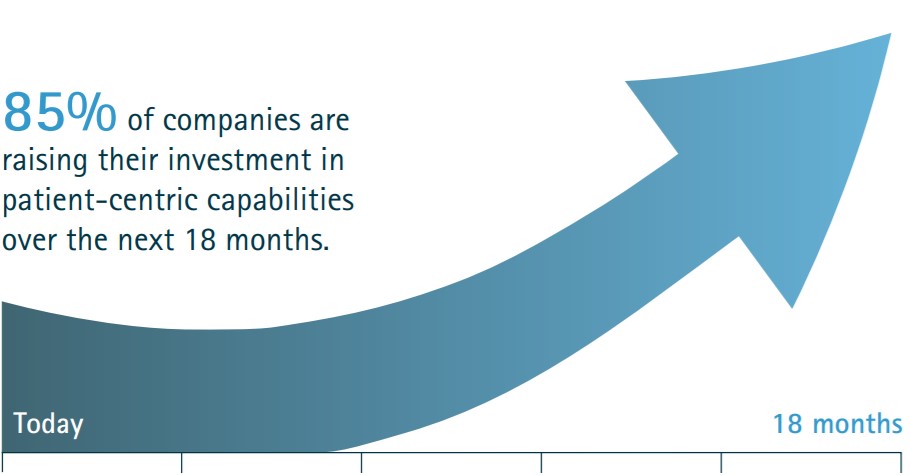
Patient programs for improved patient outcomes
In the digital age of present, patients have never been better equipped with the devices and technologies to take control of their own healthcare needs and outcomes….

Are you ready for the mandatory transition to eCTD for prescription medicines?
A staged implementation has been proposed for prescription medicines to reflect the current industry uptake of the eCTD format. The transition begins with new chemical entity, new biological entity, biosimilar…
Making the most of complementary medicines reforms
In March this year the TGA finally started rolling out the long awaited Complementary Medicine…