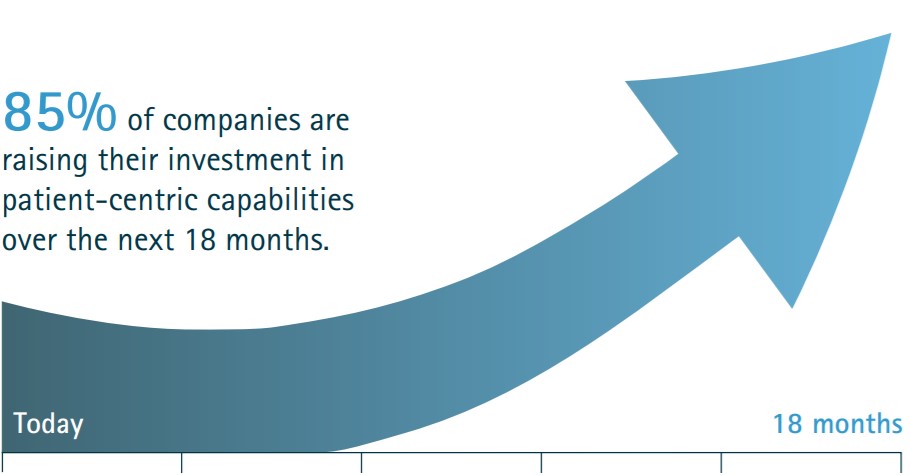
Brexit – What’s the impact on medicines and medical devices supplied in Australia?
A transition period is now in effect until the end of 2020 with the possibility for extension. Its status quo for Australia during the transition period, with the current regulations for…