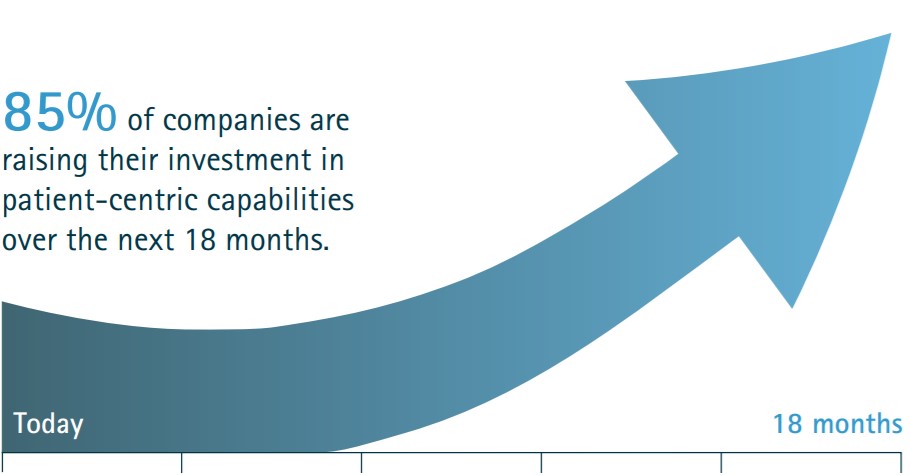
How will the new Strategic Agreements impact your business?
Medicines Australia and Generic and Biosimilars Association have secured new 5 year agreements with the Commonwealth for timely access to and reimbursement of medicines and policy settings that recognise the...