The effectiveness of one intervention compared to another, also known as the treatment effect, is frequently a key consideration when assessing the cost-effectiveness of alternative therapeutic interventions for economic evaluations.
Therefore, it is crucial that these characteristics are correctly identified and calculated. Multiple drug alternatives are becoming available in most therapeutic areas, but frequently there isn’t enough data from head-to-head clinical studies (Figure 1A) to compare the efficacy (and/or safety) of one therapy with another. Indirect comparisons between or among different medications can be used to elucidate relative efficacies between drugs in the absence of head-to-head clinical trial data.
Indirect treatment comparison (ITC)
Treatments that have not been directly compared to one another in a randomised trial can be compared using an indirect treatment comparison (ITC). This method entails the simultaneous analysis of indirect evidence from RCTs comparing treatments of interest with a common comparator (Figure 1B). For instance, if treatments A and B have been compared with a common treatment, C, in two different sets of trials [A vs. C and B vs. C]), the relative effectiveness of treatments A and B can be estimated indirectly via the common comparator C, preserving the within-trial randomisation (a process called adjusted indirect comparison).
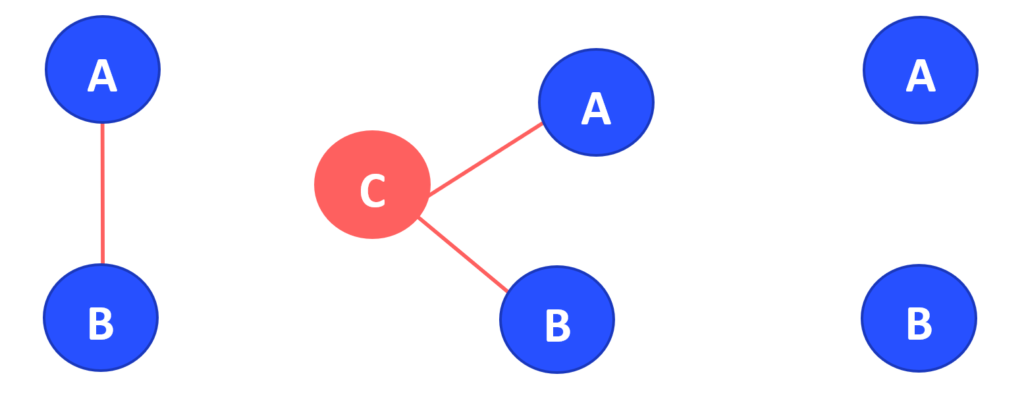
The sets of AC and BC studies must have uniform distributions of effect modifiers for an indirect comparison (such as AB) to be valid. Such effect modifiers include or example, treatment dose, severity of illness, or sample size. Only when this is the case can we infer that the intervention effects are transitive, extending clinical and methodological homogeneity to comparisons between groups of studies that compare treatments.
The role of ITC in Health Technology Assessment
Drug reimbursement organisations including the Australian Pharmaceutical Benefits Advisory Committee (PBAC), recognise adjusted indirect comparison as an accepted method. The PBAC guidelines provide a set of principles to use indirect comparisons of additional analyses trial results as part of the clinical evaluation (Section 2). The guidelines describe the Bucher single pairwise method as the most widely used, since it allows to “indirectly compare the odds ratios from randomised trials that share a common reference arm”. Furthermore, the provide guidance on the key steps in assessing the transitivity assumption for indirect comparisons and the heterogeneity of comparative treatment effects.
Despite the acceptability of indirect treatment comparison, not taking randomisation into account, such as using different treatment arms as though they were from the same trial (also known as naive indirect comparison) (Figure 1C), or indirect comparisons where variations in trial characteristics may affect the transitivity of the trials in the comparison, are difficult to interpret and lower the confidence of decision-making from PBAC.
For instance, in previous rejections of submissions using indirect comparison, the PBAC has noted several issues, including the representation of the treatment effect in terms of point estimates rather than confidence intervals and the exclusion of dose adjustment in trials published after the PBAC’s review was completed. Transitivity issues have also been identified by the PBAC when different treatment regimens were compared, which have led to rejection as they did not adequately demonstrate non-inferiority between the proposed drug and the nominated comparators.
Most of these issues can be addressed by ensuring homogeneity of the of the trial outcomes being compared. This can include clarifying the treatment arm that was used for each trial in the analysis, using the same exposure metric across trials and having similar baseline patient characteristics.
How Commercial Eyes can support sponsors
The Commercial Eyes Access, Research & Intelligence (ARI) team consists of experienced consultants, including specialists in market access, market research and market intelligence. We offer a holistic approach to new product planning, market assessment and developing reimbursement and pricing strategies and solutions for medicines including cell and gene therapies, devices and diagnostics. Our consultants have a practical and thorough understanding of the Australian healthcare environment backed by sound technical skills, knowledge and experience.
Our Access, Research & Intelligence team can provide an end-to-end service throughout the life cycle of your technology and can help you navigate Australia’s sophisticated reimbursement, policy and legal framework for the pricing of pharmaceuticals, nutritional products, blood products, vaccines, devices and prosthetics. Contact our experienced team at [email protected]
References
Ahuja, Dhruva; Singh, Siddharthb,c. Comparative efficacy trials in inflammatory bowel disease: current and future implications for practice. Current Opinion in Gastroenterology: July 2022 – Volume 38 – Issue 4 – p 337-346 doi: 10.1097/MOG.0000000000000854
Cipriani, A. , Higgins, J. P. , Geddes, J. R. & Salanti, G. (2013). Conceptual and Technical Challenges in Network Meta-analysis. Annals of Internal Medicine, 159 (2), 130-137.
Drummond, Michael F., et al. Methods for the Economic Evaluation of Health Care Programmes, Oxford University Press, 2015.
Kim H, Gurrin L, Ademi Z, Liew D. Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data. Br J Clin Pharmacol. 2014 Jan;77(1):116-21. doi: 10.1111/bcp.12150. PMID: 23617453; PMCID: PMC3895352.
Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (PBAC). Version 5.0, September 2016. Available at: https://pbac.pbs.gov.au/

