In the last 12 months, Commercial Eyes has enrolled over 8000 patients into its various patient programs, addressing a diverse range of needs and backgrounds. These programs are designed to support both patients and healthcare professionals (HCPs) by improving access to and understanding of essential treatments. Each program is designed with a thorough understanding of patient needs and the specific stage of market access for the therapy in question.
Commercial Eyes works with clients to design, develop, and deliver a variety of programs to ensure that every patient receives the best possible care and support throughout their treatment journey. These programs aim to balance the need for timely access to potentially life-saving treatments with the requirement to ensure patient safety and the proper use of therapeutic goods. In the wider healthcare context, these patient programs play a crucial role in enhancing patient outcomes, building trust in pharmaceutical brands, and tackling healthcare disparities.
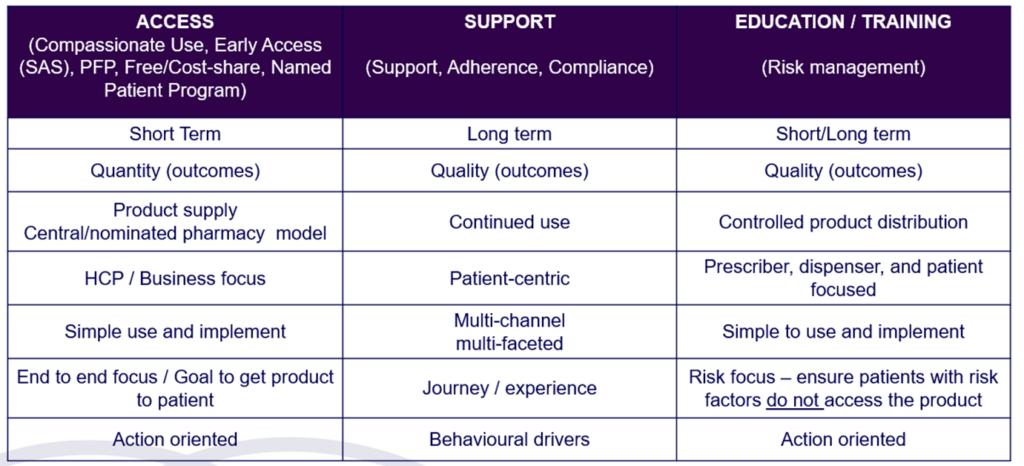
Access Program / Familiarisation Program (PFP)
Access Program/ Familiarisation Program (PFP) is a structured initiative by pharmaceutical companies to introduce HCPs to new medications that are pending regulatory approval or are newly approved but not reimbursed by the government. These programs aim to educate HCPs about the proper use, benefits, and potential risks of the new products, allowing them to gain practical and clinical experience and confidence in prescribing these medications once they become commercially available.
Access Programs are beneficial for HCPs to provide clinical experience and become familiar with the treatment and for patients to access potentially life-changing medications before they become widely available or reimbursed.

At Commercial Eyes, we develop comprehensive and compliant frameworks for enrollment, continuations, grandfathering (transitioning to commercially available product), and logistics management to create the most effective access programs for our clients. Our goal is to ensure that patients receive timely access to essential medications while maintaining high standards of safety.
Key aspects of access programs include:
- Education and Training: Providing detailed information and training to HCPs about the new medication, including its indications, dosing, administration, and potential side effects.
- Patient Access: Allowing a selected group of patients to access the medication under the supervision of participating HCPs. This helps to gather real-world data and experiences on the drug’s effectiveness and safety.
- Data Collection: Data on the medication’s performance in a real-world setting can be valuable for regulatory submissions and post-marketing surveillance.
Compassionate Access Program (CAP)
A CAP is designed to provide patients with access to registered medicines, or
products that require dose increases not reimbursed by the government.
At Commercial Eyes, we specialise in developing and managing Compassionate Access Programs, ensuring that patients receive the medications they need while complying with all regulatory requirements. In our deep dive into CAPs we explore some recent examples of well-designed CAPS to achieve company objectives for patient access.
Key aspects of a Compassionate Access Program include:
- A detailed patient eligibility criteria.
- Stakeholder relationship management with logistic partners to ensure supply and distribution of the product to patients as per the required adherence schedule.
- Documentation and Reporting: Maintenance of detailed records of the patient’s treatment, response, and any side effects reported as required by regulatory authorities. This information can be crucial for future regulatory submissions and approvals.
- May also include a co-payment for the product managed through our internal payment portal or third party logistics provider
Managed Access Program (MAPs)
A Managed Access Program (MAP), run by pharmaceutical companies is a specialised program designed to provide access to medications for patients who may not meet traditional approval or government reimbursement criteria.
At Commercial Eyes, we specialise in running MAPs on behalf of pharmaceutical companies. We embed ourselves within our client’s processes to ensure seamless integration and efficient execution of the MAP. Our comprehensive approach includes managing patient enrollment, ensuring regulatory compliance, coordinating logistics, and providing ongoing support. Within our Managed Access program, we can work within our client’s system and with its organisation for internal approval. We view ourselves as an extension of their team.

Special Access Scheme (SAS) Program
SAS is a pathway designed to provide HCPs the ability to prescribe their patients medicines, medical devices, or biologicals that are not yet registered or approved by the Therapeutic Goods Administration (TGA) in Australia but may be required for the treatment of patients with rare, serious or life-threatening conditions or when conventional treatments have failed, are unsuitable, or are unavailable.
SAS program can be run in conjunction with a CAP or Access program and may operate for a short period, multiple years, or within a predetermined timeframe.
At Commercial Eyes, we collaborate with our clients to simplify SAS programs. We co-create SAS program workflows, manage internal approvals and SAS product logistics, and assist HCPs with form management.
The SAS operates under specific conditions:
- Category A: For patients who are seriously ill or have a condition that could lead to death without early treatment. This category allows immediate access without prior TGA approval, but the TGA must be notified within four weeks.
- Category B For patients who do not meet Category A criteria. In this case, a medical practitioner must apply to the TGA for approval before the patient can access the therapeutic goods.
- Category C: Covers a list of specified medicines and medical devices that have an established history of use, where the TGA has determined the risk-benefit profile is acceptable for broader access. Medical practitioners must notify the TGA of each use.
Patient Support Program (PSP)

At Commercial Eyes, we’re committed to creating Patient Support Programs that prioritise long-term sustainability and truly place patients at the centre. Medical treatments can be complex and, at times, overwhelming, so our goal is to offer comprehensive support every step of the way, particularly when their treatment journey may be ongoing.
The key priority for PSPs is to build eco-systems that support patients and ensure continuity over time. By focusing on sustainability, we aim to foster a supportive environment that benefits both patients and therapy brands for the long term. Through our PSPs patients can feel empowered and connected to the resources they need, helping them achieve better health outcomes and an improved treatment experience.
By blending advanced healthcare services with a compassionate, human touch, we ensure that patients receive not only essential medical education and care but also the emotional and psychological support that makes a real difference. Importantly, we can adapt the program services over time as we take on board patient and carer feedback about what helps most. This holistic approach supports long-term sustainability by addressing both the practical and emotional aspects of treatment, making the journey more positive and effective for each patient. Some of the key service offerings in our PSPs are:
- Telehealth Services: One of the standout features of our Patient Support Program is our team of internal nurses who conduct telehealth sessions. These experienced HCPs are available to offer guidance, answer questions, and provide emotional support to patients from the comfort of the patient’s homes.
Our Telehealth services ensure that patients have access to medical advice and support at their convenience, enhancing their treatment experience
- Infield Nurses: In addition to our Telehealth services, we have an in-field nurse support that Commercial Eyes can draw upon for support, such as injection training and other hands-on assistance. These nurses are skilled in providing personalised training to patients, ensuring they feel confident and capable of managing their treatments.
- Prescription reminder services: allows patients to schedule reminders for collection of their prescriptions from their HCP or from their pharmacy.
- Additional resources – providing access and information to patients about their treatment journey whilst leveraging to complement valuable services and resources provided by existing patient foundations and forums.
- Electronic Direct Mail(s) (EDMs) – curated information provided to the patient at timely intervals during their treatment journey, helping to ensure adherence and provide support.
Risk Management Program to support Risk Management Plan (RMP)
A Risk Management Plan (RMP) in Australia is a comprehensive safety plan required by the Therapeutic Goods Administration (TGA) as part of the approval process for some medicines and medical devices. The RMP document outlines how risks associated with the use of a product or device will be identified, assessed, and managed throughout the product/device lifecycle. The aim of an RMP is to ensure that any potential risks to patients are minimised and that the benefits of the product outweigh these risks. Our Risk Management Programs are designed to support the implementation of the RMP. ome of the key service offerings in our RMP include:
Assessment and Efficiency Mapping: We continuously assess the effectiveness of the RMP and map out efficiencies to ensure that the plan is achieving its intended goals.
Material Creation: We develop and create all necessary educational and informational materials to support the RMP, tailored to the needs of both HCPs and patients.
Platform Creation and Validation: We design and build platforms to manage the RMP, including validation processes (if required), ensuring they meet regulatory standards and are fit for purpose.
Program Implementation: We manage the entire RMP, ensuring that patients receive the necessary training and support to use the product safely and effectively, or to ensure that any required tests are undertaken prior to treatment.
Collaboration with HCPs and Pharmacists: We work closely with healthcare professionals and pharmacists to ensure they are fully enrolled in the program and that products are being dispensed correctly through the RMP.
Pharmacovigilance: Our team monitors and evaluates the safety of the product, identifying and addressing any adverse events or safety concerns.

In Australia, RMPs are mandatory for new medicines, biologicals, and some medical devices. They may also be required for products already on the market if new safety concerns arise. The TGA reviews and approves the RMP, and the plan must be updated as new information becomes available, ensuring ongoing management of any risks associated with the product.

