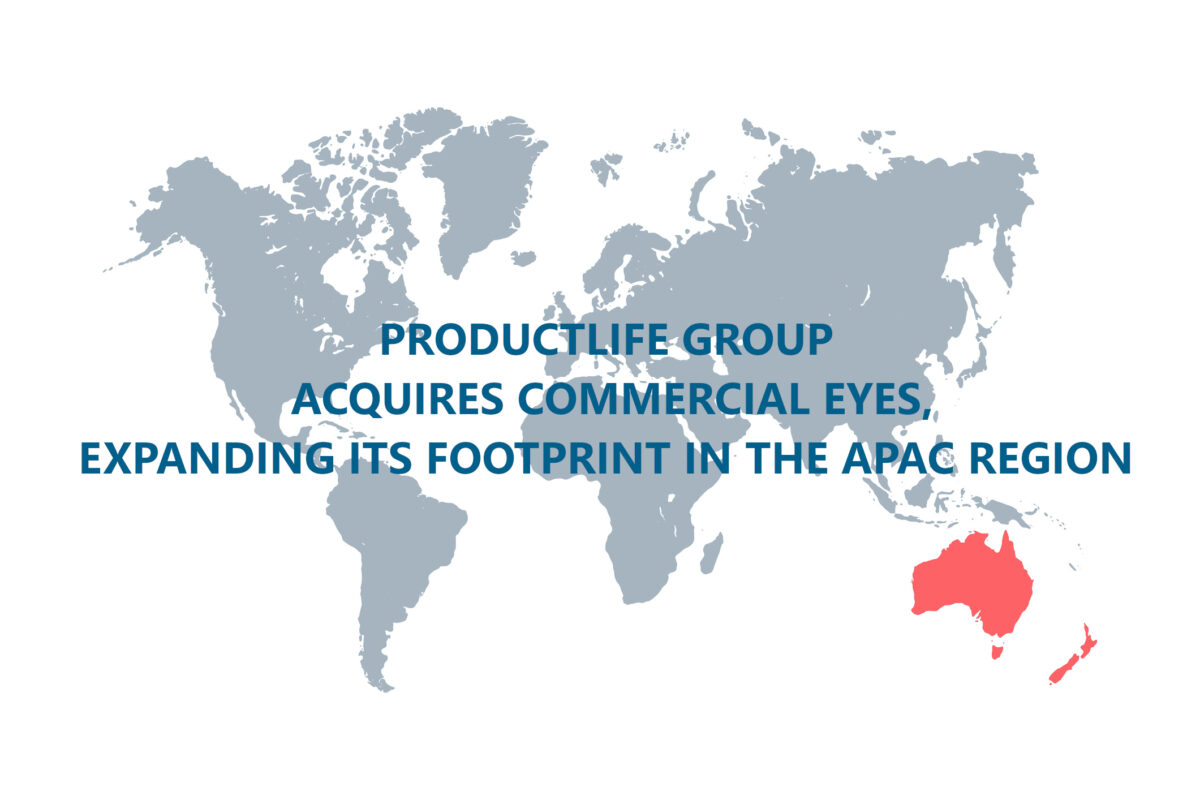
Productlife Group Acquires Commercial Eyes, Expanding Its Footprint In The APAC Region
ProductLife Group expands its expertise in APAC through the acquisition of Commercial Eyes, Australia’s leading pharmaceutical and medical device commercialisation company, specialising in regulatory affairs, pharmacovigilance, medical information, quality assurance,…